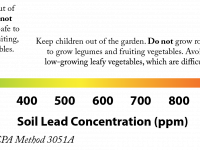
Chemical properties of soils include the following aspects: inorganic matters of soil, organic matters in soil, colloidal properties of soil particles and soil reactions and buffering action in acidic soils and basic soils. The chemical side of a soil is extremely important of course and is about the correct balance of the available nutrients in the soil. This is largely determined by the organic-matter content and its humus percentage; this is the ‘store house’ of nutrients on any farm. The extent to which minerals have a dominant presence or not, affects the release of specific nutrients. Supplementing shortages is important, but the right balance is even more important. The soil only produces nutrients if you have the right balance. Chemical and physical properties impact biological properties. Optimal chemical and physical properties will lead to optimal biological properties and soil functions i.e. nutrient and water cycling.
| Title | Source | Resource type and Date | Short Summary / Preview |
|---|---|---|---|
| Nutrient Management: An Introduction | SARE | Book Excerpt |
Soil organic matter encompasses all non-mineral solids in soil, arising from biological tissues, byproducts, and wastes.
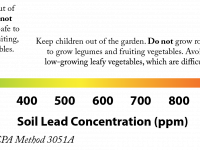
A soil contaminant is any substance that exceeds naturally occurring-levels and poses human health risks.
Essential plant nutrients required in very small amounts that are needed to sustain plant growth and development.

The carbon-to-nitrogen ratio in soil is the ratio of the mass of carbon-to-nitrogen.

Soil salinity is caused by excessive levels of water soluble salts in the soil water.
Secondary nutrients are nutrients that slightly limit crop growth and are moderately required by plants.
Soil sodicity is caused by excessive saturation of sodium (Na + ) ions at the soil cation exchange sites, which is measured by Sodium Adsorption Ratio (SAR).
Cation exchange capacity (or CEC) is a measure of a given soil’s capacity to retain or store positively charged ions. Base saturation is the percentage of the CEC occupied by basic cations (Ca 2+ , Mg 2+ , K + and Na + ).
Potassium is an essential plant nutrient used in key intracellular processes for supporting plant growth, including sugar and nutrient transport, stomatal regulation, photosynthesis, and acting as a catalyst for plant enzymatic processes.
Phosphorus is a nutrient required in plants for several complex functions such as energy transformation, photosynthesis, nutrient movement, sugar and starch transformation, and genetic transfer.
Nitrogen is an essential plant nutrient needed for growth, development, and reproduction that is a critical component of organic molecules such as proteins, amino acids, and nucleic acids. It may be found in the soil in mineralizable (organic) or plant available (mineralized) forms.
Soil pH is an indicator of how acidic, neutral or alkaline (basic) a soil is, based on the hydrogen ion concentration – where pH is reported as a negative logarithm and ranges from 0-14.
Soil EC is a measure of the concentration of ions from water-soluble salts in soils, and the test results are indicative of soil salinity.